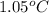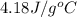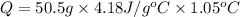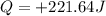Chemistry, 03.09.2019 01:30 ToriChristine
A0.500-g sample of kcl is added to 50.0g of water in a calprimeter (figure 5.12) if the temperature decreases by 1.05c. what is the approximate amount of heat involved in the dissolution of the kcl , assuming the heat capacity of the resulting solution is 4.18 j/gc? is the reaction exothermic or endothermic?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1
You know the right answer?
A0.500-g sample of kcl is added to 50.0g of water in a calprimeter (figure 5.12) if the temperature...
Questions


History, 16.07.2019 15:30

History, 16.07.2019 15:30

History, 16.07.2019 15:30


Biology, 16.07.2019 15:30

Mathematics, 16.07.2019 15:30


History, 16.07.2019 15:30

Biology, 16.07.2019 15:30

Biology, 16.07.2019 15:30


History, 16.07.2019 15:30

Mathematics, 16.07.2019 15:30




Social Studies, 16.07.2019 15:30


Biology, 16.07.2019 15:30

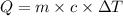
 = change in temperature =
= change in temperature =