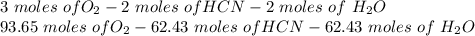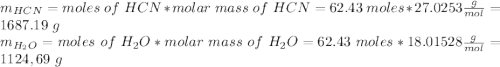Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane: 2nh3 1g2 1 3o2 1g2 1 2ch4 1g2 h 2hcn1g2 1 6h2o1g2 if 5.00 3 103 kg each of nh3, o2, and ch4 are reacted, what mass of hcn and of h2o will be produced, assuming 100% yield?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane:...
Questions

Mathematics, 10.03.2021 01:00




Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Biology, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

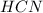 and 1124,69 g of
and 1124,69 g of 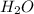
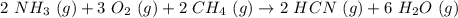
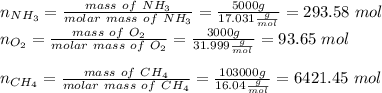
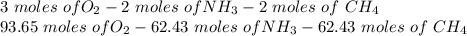
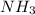 and
and 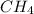 to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.
to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.