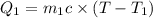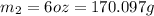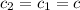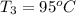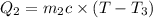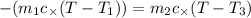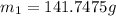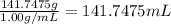Chemistry, 03.09.2019 01:20 emilybomar7466
How many millilitres of water at 23 degree centigrade with the density of 1.00 g / ml must be mixed with 180 ml (about 6 oz) of coffee at 95 degree centigrade so that the resulting combination will have a temperature of 60 degree centigrade assume that coffee and water have the same density and the same specific heat

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3


Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
How many millilitres of water at 23 degree centigrade with the density of 1.00 g / ml must be mixed...
Questions




History, 17.11.2020 17:00

Physics, 17.11.2020 17:00







Mathematics, 17.11.2020 17:00


Social Studies, 17.11.2020 17:00

Mathematics, 17.11.2020 17:10




Mathematics, 17.11.2020 17:10

Health, 17.11.2020 17:10

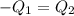
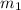
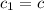
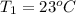
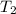 = 60 °C
= 60 °C