Chemistry, 03.09.2019 00:10 thedragontale1020
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020 m) ag if e (h) - +0.000 v and e(ag)-0.799 v. a. +0.817 v b. +0.799 v c. +0.911 v d. +0.275 v e. +1.01 v ,

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020...
Questions




Computers and Technology, 31.08.2019 02:30

Mathematics, 31.08.2019 02:30


Physics, 31.08.2019 02:30


History, 31.08.2019 02:30

Advanced Placement (AP), 31.08.2019 02:30



Social Studies, 31.08.2019 02:30


Computers and Technology, 31.08.2019 02:30



Biology, 31.08.2019 02:30


![E^0_{[H^{+}/H_2]}=+0.00V](/tpl/images/0221/4856/30abe.png)
![E^0_{[Ag^{+}/Ag]}=+0.799V](/tpl/images/0221/4856/2c999.png)
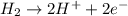
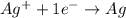
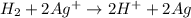
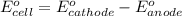
![E^o_{cell}=E^o_{[Ag^{+}/Ag]}-E^o_{[H^{+}/H_2]}](/tpl/images/0221/4856/e75b5.png)