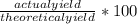Freon-12 ccl2f2, is prepared from ccl4 by reaction with hf. the other product of this reaction is hcl. outline the steps needed to determine the prcent yield of a reaction that produces 12.5 g of ccl2f2 from 32.9 g of ccl4. feron-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone an d has a very long lifetime in the atmosphere. determine the percent yield.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Freon-12 ccl2f2, is prepared from ccl4 by reaction with hf. the other product of this reaction is hc...
Questions


Chemistry, 25.09.2020 03:01

English, 25.09.2020 03:01

Mathematics, 25.09.2020 03:01






Geography, 25.09.2020 03:01


Mathematics, 25.09.2020 03:01

History, 25.09.2020 03:01




English, 25.09.2020 03:01


History, 25.09.2020 03:01

Mathematics, 25.09.2020 03:01