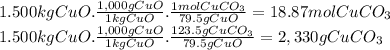Chemistry, 02.09.2019 22:10 BlueExorcistReaper
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to exercise 4.42.
write the balanced equation, then outline the steps necessary determine the information equation in each of the following:
(a) the number of moles than the mass of the chlorine cl2 required to react with 10.0 g of sodium metal na to produce sodium chloride nacl
(b) the number of moles and the mass of the oxygen formed by the decomposition of 1.252 gram of mercury (ii) oxide
(c)the number of the moles and the mass of the sodium nitrate nano3 required to produce 128 gram of oxygen (nano2 is the other product)
(d)the number of moles and the mass of the carbon dioxide formed by the combustion of 20.0 kg of carbon in excess of oxygen
(e)the number of moles and the mass of the copper(ii) carbonate needed to produce 1.500 kg of copper ii oxide (co2 is the other product)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to...
refer to...
Questions

Mathematics, 13.01.2021 19:40


Mathematics, 13.01.2021 19:40

Mathematics, 13.01.2021 19:40


Mathematics, 13.01.2021 19:40








Physics, 13.01.2021 19:40



Mathematics, 13.01.2021 19:40

Arts, 13.01.2021 19:40

Advanced Placement (AP), 13.01.2021 19:40

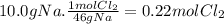
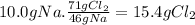
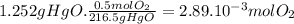
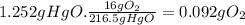
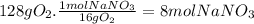
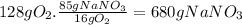
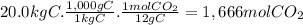
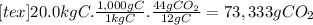 " alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />
" alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />