
Chemistry, 02.09.2019 21:10 lekaje2375
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and oxidising and reducing agents in each of the following equations:
(a)mg + nicl2 -> mgcl2 + ni
(b)pcl3 + cl2 -> pcl5
(c)c2h4 + 3o2 -> 2co2 + 2h2o
(d)zn + h2so4 -> h2 + znso4
(e)2k2s2o3 + i2- > k2s4o6+2ki
(f)3cu + 8hno3 = 3cu(no3)2 + 4h2o + 2no

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

You know the right answer?
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and ox...
Questions

Mathematics, 01.11.2019 21:31




Chemistry, 01.11.2019 21:31

English, 01.11.2019 21:31


Mathematics, 01.11.2019 21:31




Mathematics, 01.11.2019 21:31



Biology, 01.11.2019 21:31

History, 01.11.2019 21:31

Biology, 01.11.2019 21:31


History, 01.11.2019 21:31

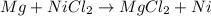
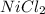 acts as oxidizing agent.
acts as oxidizing agent.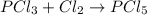
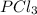 acts as reducing agent and
acts as reducing agent and 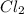 acts as oxidizing agent.
acts as oxidizing agent.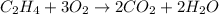
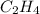 acts as reducing agent and
acts as reducing agent and 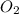 acts as oxidizing agent.
acts as oxidizing agent.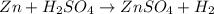
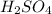 acts as oxidizing agent
acts as oxidizing agent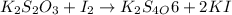
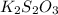 acts as reducing agent and
acts as reducing agent and 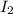 acts as oxidizing agent.
acts as oxidizing agent.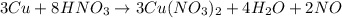
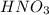 acts as oxidizing agent
acts as oxidizing agent


