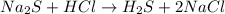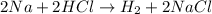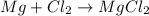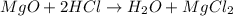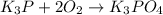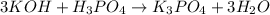Chemistry, 02.09.2019 20:30 makaylarae8781
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl -> h2s + 2nacl
(b)2na + 2hcl -> h2 + 2nacl
(c)mg + cl2 = mgcl2
(d)mgo + 2hcl = h2o + mgcl2
(e)k3p+2o2 -> k3po4
(f)3koh +h3po4 -> k3po4 + 3h2o

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3


Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl...
(a)na2s + hcl...
Questions


Business, 22.04.2021 16:20



Mathematics, 22.04.2021 16:20

History, 22.04.2021 16:20



History, 22.04.2021 16:20


Mathematics, 22.04.2021 16:20


History, 22.04.2021 16:20



Mathematics, 22.04.2021 16:20

Mathematics, 22.04.2021 16:20