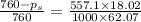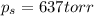Chemistry, 02.09.2019 20:20 genyjoannerubiera
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene glycol (molar mass g/mol). the vapor pressure of pure water at t00.0 °c is 760 torr. (2 points) 62.07 g/mol) in 1000.0 g of water (h20 molar mass 18.02 a) 106 torr b) 0.756 torr c) 760 torr (d)186 torr e) none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene gl...
Questions



History, 24.06.2019 15:40












English, 24.06.2019 15:40


Chemistry, 24.06.2019 15:40

Mathematics, 24.06.2019 15:40


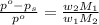
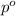 = vapor pressure of pure solvent (water) = 760 torr
= vapor pressure of pure solvent (water) = 760 torr 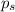 = vapor pressure of solution = ?
= vapor pressure of solution = ?
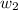 = mass of solute (ethylene glycol) = 557.1 g
= mass of solute (ethylene glycol) = 557.1 g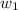 = mass of solvent (water) = 1000.0 g
= mass of solvent (water) = 1000.0 g
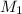 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole
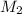 = molar mass of solute (ethylene glycol) = 62.07 g/mole
= molar mass of solute (ethylene glycol) = 62.07 g/mole