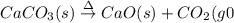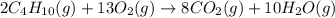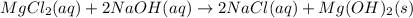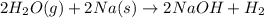Write a balanced molecular equation describing each of the following chemical reactions:
(a)s...

Chemistry, 02.09.2019 20:10 codyshs160
Write a balanced molecular equation describing each of the following chemical reactions:
(a)solid calcium carbonate is heated and decomposes to sold calcium oxide and carbon dioxide
(b)gaseous butane, c4h10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor
(c)aaqeous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride
(d)water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
You know the right answer?
Questions


Mathematics, 29.08.2019 05:30







History, 29.08.2019 05:30



Mathematics, 29.08.2019 05:30


Mathematics, 29.08.2019 05:30

Physics, 29.08.2019 05:30

Biology, 29.08.2019 05:30

Social Studies, 29.08.2019 05:30

Mathematics, 29.08.2019 05:30

Mathematics, 29.08.2019 05:30