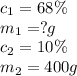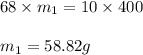Chemistry, 02.09.2019 19:10 DaylaReevaFEEVA2757
Consider this question: what mass of concentrated solution nitric acid( 68.0% hno3 by mass) is needed to prepare 400.0 g of a 10.0% solution of hno3 by mass?
(a) outline the steps necessary to answer the question
(b) answer the question

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Consider this question: what mass of concentrated solution nitric acid( 68.0% hno3 by mass) is need...
Questions

History, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31



Mathematics, 07.12.2019 01:31



Mathematics, 07.12.2019 01:31

Social Studies, 07.12.2019 01:31





World Languages, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31


Mathematics, 07.12.2019 01:31

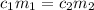
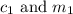 are the concentration and mass of concentrated solution.
are the concentration and mass of concentrated solution. are the concentration and mass of diluted solution.
are the concentration and mass of diluted solution.