
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solution, a concentrated hcl solution
(b)2.026 g in 0.1250 ml of a solution used as an unknown in general chemistry laboratory
(c) 0.001 mg cd2+ in 0.100 l the maximum permissible concentration of cadmium in drinking water
(d) 0.0079 g c7h5sno3 in one ounce(29.6ml), concentration of saccharin in a diet soft drink

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solu...
(a) 293 gram hcl in 666 ml of solu...
Questions




















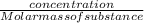
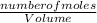
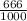 dm³ , 0.666dm³
dm³ , 0.666dm³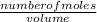
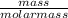
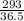 = 8.03mole
= 8.03mole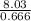 = 12.06moldm⁻³
= 12.06moldm⁻³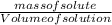 =
= 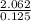 = 16.496g/mL
= 16.496g/mL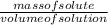 =
= 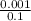 = 0.01mg/L
= 0.01mg/L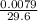 = 0.00027g/mL
= 0.00027g/mL

