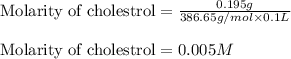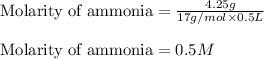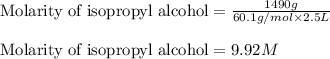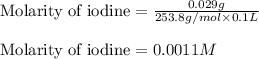Chemistry, 02.09.2019 18:30 allimaycatp8qgaq
Calculate the molarity of each of the following solutions:
(a) 0.195 g of cholestrol, c27h46o, in 0.100 l of serum, the average concentration of cholestrol in human serum
(b) 4.25 gram of nh3 in 0.500 l solution, the concentration of nh3 in household ammonia
(c) 1.49 kg of isopropyl alcohol c3h7oh in 2.50 l of solution the concentration of isopropyl alcohol in rubbing alcohol
(d)0.029 gram of i2 in 0.100 l of solution the solubility of i2 in water at 20 c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

You know the right answer?
Calculate the molarity of each of the following solutions:
(a) 0.195 g of cholestrol, c27h46o...
(a) 0.195 g of cholestrol, c27h46o...
Questions






Law, 01.12.2020 16:40

Mathematics, 01.12.2020 16:40









History, 01.12.2020 16:40




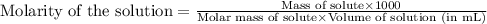 .....(1)
.....(1)