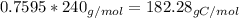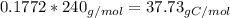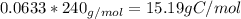Chemistry, 02.09.2019 18:10 destinyd10189
Amajor textile dye manufacturer developed new yellow dye. the dye has a percent composition of 75.9 5% c, 17.72% n
and 6.33% h by mass with the molar mass of about to 240 g/mol. determine the molecular formula of the dye.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
Amajor textile dye manufacturer developed new yellow dye. the dye has a percent composition of 75.9...
Questions


Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20


Chemistry, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

Social Studies, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Physics, 18.03.2021 02:20