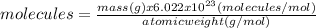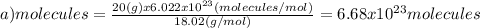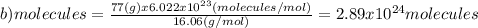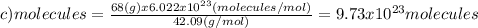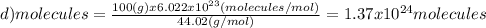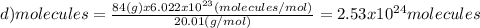Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)...

Chemistry, 02.09.2019 17:30 angelrgomez01
Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)
(b)77.0 g of ch4 (16.06 g/mol)
(c)68.0 g of cah2 (42.09 g/mol)
(d)100.0 g of n2o (44.02 g/mol)
(e)84.0 g of hf (20.01 g/mol)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Questions



Mathematics, 05.05.2021 18:10


Biology, 05.05.2021 18:10

Mathematics, 05.05.2021 18:10



English, 05.05.2021 18:10


Mathematics, 05.05.2021 18:10



Computers and Technology, 05.05.2021 18:10

Mathematics, 05.05.2021 18:10

History, 05.05.2021 18:10




Computers and Technology, 05.05.2021 18:10