Chemistry, 02.09.2019 16:30 carlinryan
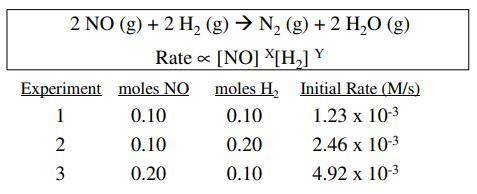
The reaction of nitric oxide with hydrogen at 1280 c is 2no(g) +2h2(g)n2(g)+2h2o(g) from the following data collected at this temperature, determine the rate law and calculate the rate constant. initial rate (m/s) 1.3 x 10 5.0 x 10 experiment 1 2 3 no 0050 0100 0100 .0020 .0020 0040 10.0 x 105

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
The reaction of nitric oxide with hydrogen at 1280 c is 2no(g) +2h2(g)n2(g)+2h2o(g) from the followi...
Questions

Physics, 19.11.2021 17:20



English, 19.11.2021 17:20

English, 19.11.2021 17:20

English, 19.11.2021 17:20

Biology, 19.11.2021 17:20







Spanish, 19.11.2021 17:30

Biology, 19.11.2021 17:30


Mathematics, 19.11.2021 17:30



English, 19.11.2021 17:30