
Chemistry, 02.09.2019 16:20 Justus4215
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are the concentrations of a, b, and c at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 m, 0.40 m, and 0 m, respectively?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are...
Questions

Mathematics, 26.05.2021 18:30

English, 26.05.2021 18:30



Mathematics, 26.05.2021 18:30

Physics, 26.05.2021 18:30


Chemistry, 26.05.2021 18:30












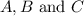 at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.
at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.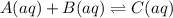
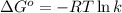
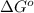 = standard free‑energy change = -4.20 kJ/mole
= standard free‑energy change = -4.20 kJ/mole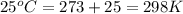
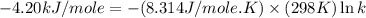
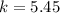
![k=\frac{[C]}{[A][B]}](/tpl/images/0220/8877/b93eb.png)
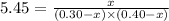
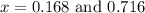
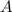 at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M
at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M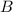 at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M
at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M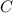 at equilibrium = x = 0.168 M
at equilibrium = x = 0.168 M

