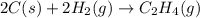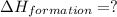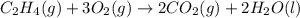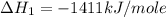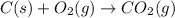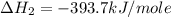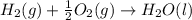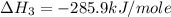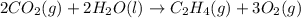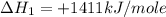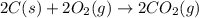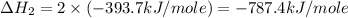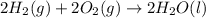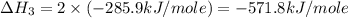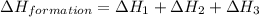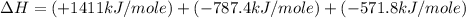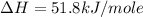Chemistry, 31.08.2019 04:10 isabella4141
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermically in an excess of air. c(s) + o2(g) → co2(g) ahⓡ =-393.7 kj mol: 1. h2(g) + % o2(g) → h2o(1) ah® --285.9 kj mol. c2h4(g) + 302(g) → 2co2(g) + 2h2o(1) ah® --1411.0 kj mol? . use the data to calculate the standard enthalpy change of formation, ah® in kj mol? , of ethene at 298 k 2c(s) + 2h2(g) → c2h4(8)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermica...
carbon, hydrogen and ethane each burn exothermica...
Questions


Spanish, 05.08.2019 01:30



English, 05.08.2019 01:30


Mathematics, 05.08.2019 01:30




Mathematics, 05.08.2019 01:30


Biology, 05.08.2019 01:30

Biology, 05.08.2019 01:30


Mathematics, 05.08.2019 01:30




Mathematics, 05.08.2019 01:30

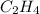 will be,
will be,