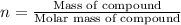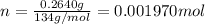Chemistry, 31.08.2019 02:30 pearpeaerrr1993
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate to titrate it to turn pink end point. the equation for this reaction is: 5na, c,o4+ 2kmno,+ 8h, so 2mnsog+ k, so,+ 5 na, so,+ 10co2+ 8h2o a) how many moles of sodium oxalate are present in the flask?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2
You know the right answer?
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate...
Questions

Mathematics, 09.02.2021 05:20

Mathematics, 09.02.2021 05:20


Spanish, 09.02.2021 05:20

Mathematics, 09.02.2021 05:20


English, 09.02.2021 05:20


Chemistry, 09.02.2021 05:20


Mathematics, 09.02.2021 05:20


Business, 09.02.2021 05:20

Mathematics, 09.02.2021 05:20

Mathematics, 09.02.2021 05:20





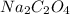 = 134 g/mol
= 134 g/mol