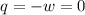Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calculate w, q and deltau for each of the following situations: (i) a reversible expansion of the sample. (ii) an irreversible expansion of the sample against a constant external pressure equal to the final pressure of the gas. (iii) a free expansion (against zero external pressure i. e. in a vacuum) of the sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

You know the right answer?
Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calcu...
Questions

Mathematics, 05.03.2021 22:20


Mathematics, 05.03.2021 22:20

Mathematics, 05.03.2021 22:20







Biology, 05.03.2021 22:30

Biology, 05.03.2021 22:30

Mathematics, 05.03.2021 22:30


Mathematics, 05.03.2021 22:30



History, 05.03.2021 22:30


Mathematics, 05.03.2021 22:30

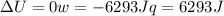
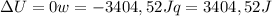
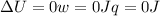
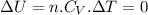 since
since 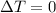
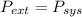 so
so 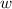 can be calculated by
can be calculated by 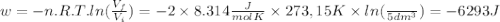
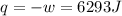
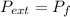
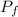 by the law of ideal gases
by the law of ideal gases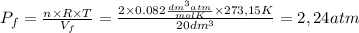
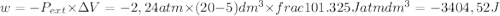
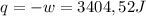
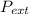 so
so 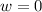 (there's no work at vaccum) and
(there's no work at vaccum) and