

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Determine how many atoms of pure silver will be created when 19.83 x 1023 atoms of copper are used i...
Questions




Mathematics, 24.05.2021 07:10


Mathematics, 24.05.2021 07:10



Mathematics, 24.05.2021 07:10

Biology, 24.05.2021 07:10


Geography, 24.05.2021 07:10


History, 24.05.2021 07:10

Mathematics, 24.05.2021 07:10

Mathematics, 24.05.2021 07:10

Mathematics, 24.05.2021 07:10


Mathematics, 24.05.2021 07:10

Chemistry, 24.05.2021 07:10

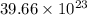
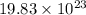
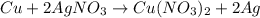
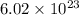 number of copper atoms react to give
number of copper atoms react to give 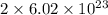 number of silver atoms.
number of silver atoms.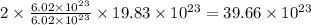 number of silver atoms.
number of silver atoms.

