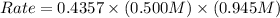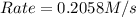Chemistry, 31.08.2019 01:00 erikamaldonado661
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what will be the initial rate (in m/s) if initial concentrations are: [a] = 0.500 m, [b] = 0.945 [m]

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what w...
Questions

History, 07.03.2020 01:42

Mathematics, 07.03.2020 01:42

English, 07.03.2020 01:42

Social Studies, 07.03.2020 01:42


Business, 07.03.2020 01:42


Mathematics, 07.03.2020 01:42



Biology, 07.03.2020 01:42









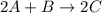
![Rate=0.4357[A][B]](/tpl/images/0213/5518/5bee5.png)