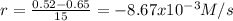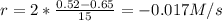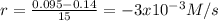a + b --> 2c

Chemistry, 30.08.2019 23:30 angsoccer02
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
concentration time data at t = 30oc
time (s) [a] (mol l-1)
0 0.65
15 0.52
30 0.35
45 0.28
60 0.22
75 0.14
90 0.095
(i) calculate the average rate of consumption of a in the first 15 seconds of reaction.
(ii) calculate the average rate of production of c in the first 15 seconds of reaction.
(iii) calculate the average rate of consumption of a in the last 15 seconds of the reaction.
(iv) explain the difference between the rates of consumption calculated in (i) and that in (iii)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

You know the right answer?
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
a + b --> 2c
Questions





Medicine, 14.12.2020 16:30


Computers and Technology, 14.12.2020 16:30






World Languages, 14.12.2020 16:30

Business, 14.12.2020 16:30

Social Studies, 14.12.2020 16:30





![r=\frac{\Delta [Concentration]}{\Delta t}](/tpl/images/0213/3224/c01cf.png)