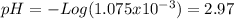Chemistry, 30.08.2019 23:30 apolloplays10
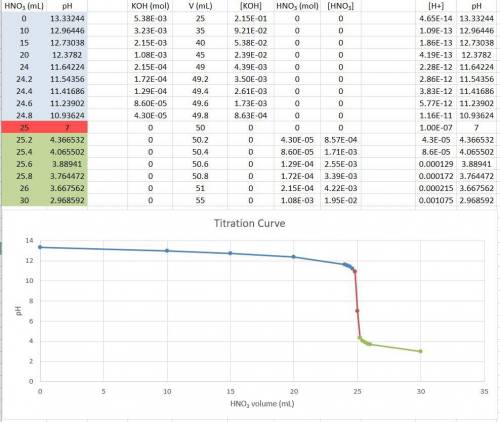
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate the volume of added acid that is required to reach equivalence point. (hint: what equals what at the equivalence point? ) b. the initial ph of koh c. calculate the ph after adding 5.0 ml of hno,. (hint: what kind of solution have you made? what equation can you use to calculate the ph? ) d. the ph after adding 10.0, 15.0, 20.0 ml e. the ph at the equivalence point f. the ph after adding 30 ml of hno g. sketch the titration curve. include labels for equivalence point, where there is excess oh and where there is excess h, o*.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate t...
Questions





Mathematics, 11.02.2020 04:44




Mathematics, 11.02.2020 04:45


Mathematics, 11.02.2020 04:45

Mathematics, 11.02.2020 04:45


Mathematics, 11.02.2020 04:45



English, 11.02.2020 04:45



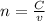
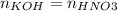
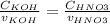
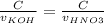
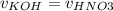
![[OH^{-} ]=\frac{moles of KOH - moles of HNO3}{total volume}](/tpl/images/0213/3384/4dd62.png)
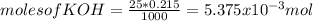
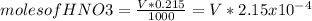
![[OH^{-} ]=\frac{5.375x10^{-3}- V*2.15x10^{-4}}{(\frac{25+v}{1000} )}](/tpl/images/0213/3384/e913a.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 5*2.15x10^{-4}}{(\frac{25+5}{1000} )}=0.143](/tpl/images/0213/3384/79893.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 10*2.15x10^{-4}}{(\frac{25+10}{1000} )}=0.0921](/tpl/images/0213/3384/e9b6e.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 15*2.15x10^{-4}}{(\frac{25+15}{1000} )}=0.0538](/tpl/images/0213/3384/ef1fb.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 20*2.15x10^{-4}}{(\frac{25+20}{1000} )}=0.0239](/tpl/images/0213/3384/6aacc.png)
![[H^{+} ]=\frac{(V-25)*2.15x10^{-4}}{\frac{V+25}{1000} }](/tpl/images/0213/3384/54d05.png)
![[H^{+} ]=\frac{(30-25)*2.15x10^{-4}}{\frac{30+25}{1000} } =1.075x10^{-3}](/tpl/images/0213/3384/583ea.png)