Hydrazine, n2h4, is a weak base and is used as fuel in the space shuttle.
n2h4(aq)+h2o(l)ân2h5...


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Questions



Mathematics, 04.12.2020 15:20



History, 04.12.2020 15:20


History, 04.12.2020 15:20

History, 04.12.2020 15:20

Biology, 04.12.2020 15:20

English, 04.12.2020 15:20

Mathematics, 04.12.2020 15:20

History, 04.12.2020 15:20

History, 04.12.2020 15:20

Mathematics, 04.12.2020 15:20



Mathematics, 04.12.2020 15:20

Mathematics, 04.12.2020 15:30

History, 04.12.2020 15:30

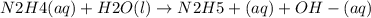
![Kb = \frac{[N2H5+][OH-]}{[N2H4]}------(1)](/tpl/images/0213/3718/63a47.png)
![[OH-] = 10^{-pOH} =10^{-3.34} =4.57*10^{-4} M](/tpl/images/0213/3718/5c095.png)
![Kb = \frac{[4.57*10^{-4}]^{2}}{[0.133]}=1.6*10^{-6}](/tpl/images/0213/3718/3b5bb.png)


