
Chemistry, 30.08.2019 22:30 ayaanwaseem
Calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. it takes 4.2 joules to change 1.0 grm (1.0 ml) of water 1.0 celcius. is it endotherrmic or exothermic? volume of vinegar 25 ml, initial temp of vinegar 17 celcius and final temp is 14 celcius.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has th...
Questions






Social Studies, 24.08.2019 03:10

Social Studies, 24.08.2019 03:10






Mathematics, 24.08.2019 03:10

History, 24.08.2019 03:10

Mathematics, 24.08.2019 03:10

Chemistry, 24.08.2019 03:10


Mathematics, 24.08.2019 03:10


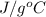
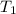 =
= 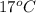 , and
, and 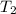 =
= 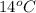
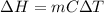
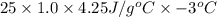 (as density =
(as density = 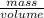 )
)

