
Chemistry, 30.08.2019 17:20 carrietaylor234
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] = 1.4 m, and kc = 11.3, what is the equilibrium concentration of c? • your answer should have two significant figures. provide your answer below:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] =...
Questions




















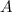 at equilibrium = 1.1 M
at equilibrium = 1.1 M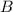 at equilibrium = 1.4 M
at equilibrium = 1.4 M
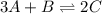
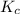 will be,
will be,![K_c=\frac{[C]^2}{[A]^3[B]}](/tpl/images/0212/4597/0cfd6.png)
![11.3=\frac{[C]^2}{(1.1)^3\times (1.4)}](/tpl/images/0212/4597/67486.png)
![[C]=4.6M](/tpl/images/0212/4597/2aa7c.png)


