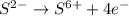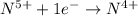Consider the following redox reaction: (38) cus + no3 → cuso4 + no2 which of the following statement is false? [1] [2] [3] cu has an oxidation state of +2 in cus. cu has an oxidation state of +2 in cuso4. s is oxidized in the above reaction. electrons are donated from s in cus to n in no3". none of the above.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

You know the right answer?
Consider the following redox reaction: (38) cus + no3 → cuso4 + no2 which of the following statemen...
Questions

Biology, 11.03.2020 05:57


Mathematics, 11.03.2020 05:57




Chemistry, 11.03.2020 05:57





History, 11.03.2020 05:58







Mathematics, 11.03.2020 05:58

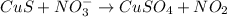
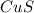 and
and 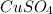 is, (+2).
is, (+2).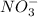 .
.