

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Abuffer consists of 0.120 m hno2 and 0.150 m nano2 at 25°c. pka of hno2 is 3.40. a. what is the ph o...
Questions





Biology, 25.11.2019 03:31



Social Studies, 25.11.2019 03:31

History, 25.11.2019 03:31


Health, 25.11.2019 03:31



Social Studies, 25.11.2019 03:31

Health, 25.11.2019 03:31



History, 25.11.2019 03:31


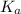 of
of 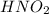 =
= 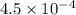 .
.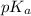 is as follows.
is as follows.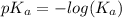
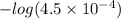
![pK_{a} + log\frac{[conjugate base]}{[acid]}](/tpl/images/0212/3783/ce755.png)
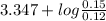
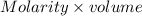

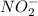 will react with
will react with 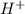 to form
to form 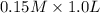
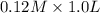
![pK_{a} + log \frac{[conjugate base]}{[acid]}](/tpl/images/0212/3783/4843d.png)


