Chemistry, 30.08.2019 16:10 derbraz6770
A250 cm^3 solution containing 1,46 g of sodium chloride is added to an excess of silver nitrate solution. the reaction is given. nacl (aq)+agno, (aq)-agci(s)+nano, (aq) what is the concentration of the sodium chloride solution? (4) 7.1 calculate the mass of the precipitate. 7.2 (4) 18

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
A250 cm^3 solution containing 1,46 g of sodium chloride is added to an excess of silver nitrate solu...
Questions


English, 05.06.2020 03:00

History, 05.06.2020 03:00






Biology, 05.06.2020 03:00

Mathematics, 05.06.2020 03:00

Mathematics, 05.06.2020 03:00


Biology, 05.06.2020 03:00







Biology, 05.06.2020 03:00

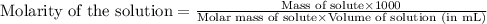
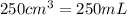
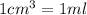
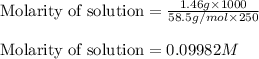
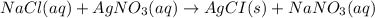
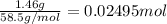
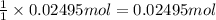 of AgCl
of AgCl