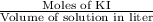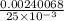Chemistry, 30.08.2019 05:30 xxaurorabluexx
The concentration of a potassium iodide solution can be determined by first adding excess silver nitrate: i'(aq) + ag+ (aq) → agl(s) the excess silver ion remaining in solution is then determined by reaction with a potassium thiocyanate (kscn) solution of known concentration: ag (aq) + scn(aq) → agscn(s) in an experiment, 50.00 ml of 0.0565 m agno3 was added to 25.00 ml of a potassium iodide solution. it then took 8.32 ml of 0.0510 m kscn solution to precipitate the unreacted silver ions. what is the concentration of the original ki solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The concentration of a potassium iodide solution can be determined by first adding excess silver nit...
Questions

Business, 20.09.2019 02:40


Mathematics, 20.09.2019 02:40

History, 20.09.2019 02:40


Business, 20.09.2019 02:40

Geography, 20.09.2019 02:40


Health, 20.09.2019 02:40

Mathematics, 20.09.2019 02:40

History, 20.09.2019 02:40




Health, 20.09.2019 02:40




Mathematics, 20.09.2019 02:40

Biology, 20.09.2019 02:40