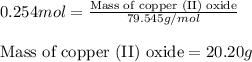Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
What is the theoretical yield in grams of copper (ii) oxide when 47.77g of copper (ii) nitrate is re...
Questions

Social Studies, 18.05.2021 20:50


Chemistry, 18.05.2021 20:50


Mathematics, 18.05.2021 20:50

Mathematics, 18.05.2021 20:50




History, 18.05.2021 20:50

Mathematics, 18.05.2021 20:50

Chemistry, 18.05.2021 20:50



Mathematics, 18.05.2021 20:50





History, 18.05.2021 20:50

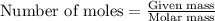 .....(1)
.....(1)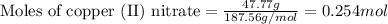
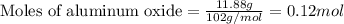
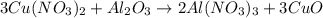
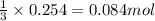 of aluminum oxide
of aluminum oxide of copper (II) oxide
of copper (II) oxide