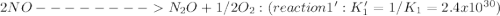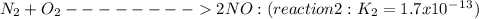Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x 10-13 find the value of the equilibrium constant for the following equilibrium reaction: n2(g) 1/202(g) n20(g) a) 7.0 x 10-44 b) 4.2 x 1017 c) 2.4 x 10-18 d) 1.6 x 109 e) 2.6 x 10-22

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x...
Questions


Mathematics, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40


Physics, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40

History, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40



Mathematics, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40

Mathematics, 08.12.2020 19:40

French, 08.12.2020 19:40



Mathematics, 08.12.2020 19:40

English, 08.12.2020 19:40