

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
For a galvanic cell that uses the following two half-reactions, cr 2o 7 2-( aq) 14 h ( aq) 6 e - → 2...
Questions

Social Studies, 11.08.2022 15:40

Mathematics, 11.08.2022 15:50



Physics, 12.08.2022 03:40

Physics, 12.08.2022 03:50


Mathematics, 12.08.2022 04:40

Physics, 12.08.2022 14:00


Social Studies, 12.08.2022 14:00




Physics, 12.08.2022 17:00

History, 12.08.2022 17:30

Mathematics, 12.08.2022 18:50

Mathematics, 12.08.2022 22:10

English, 13.08.2022 02:40

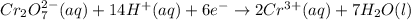
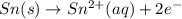
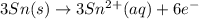
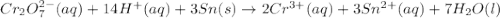
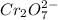 will oxidizes 3 moles of Sn
will oxidizes 3 moles of Sn moles of Sn
moles of Sn

