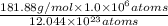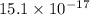Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Calculate the mass of vanadium(v) oxide (v2o5) that contains a million (1.0 *10^6) vanadium atoms. b...
Questions


English, 16.01.2022 17:20

Mathematics, 16.01.2022 17:20


English, 16.01.2022 17:20

English, 16.01.2022 17:20





Mathematics, 16.01.2022 17:30


Mathematics, 16.01.2022 17:30

Mathematics, 16.01.2022 17:30


Arts, 16.01.2022 17:30




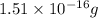
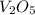 =
= 
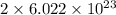 number of vanadium atom present in 1 moles of
number of vanadium atom present in 1 moles of 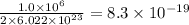 moles of
moles of 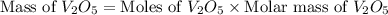
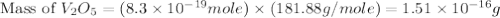
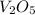 is 181.88 g/mol.
is 181.88 g/mol.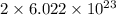 atoms
atoms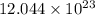 atoms
atoms vanadium atoms as follows.
vanadium atoms as follows.