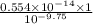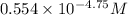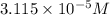Chemistry, 30.08.2019 03:00 apreston2882
You add 30ml of 1m of nh3 to a 50ml solution of 0,2 mgcl2. magnesium hydroxide (mg(oh)2) precipitates.
what is the minimum concentration of nh4+ that prevents the precipitation of mg(oh)2?
given information:
(mgoh2) ksp = 13
(nh3) kb = 10^-4.75
note: a complex formation between mg and nh4+ is ruled out.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

You know the right answer?
You add 30ml of 1m of nh3 to a 50ml solution of 0,2 mgcl2. magnesium hydroxide (mg(oh)2) precipitate...
Questions




Computers and Technology, 10.03.2020 09:00

Health, 10.03.2020 09:00

Mathematics, 10.03.2020 09:00

Chemistry, 10.03.2020 09:00


Mathematics, 10.03.2020 09:00











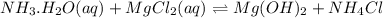
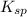 of
of 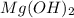 = 13 and
= 13 and 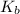 of
of 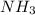 =
= 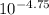
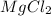 =
= 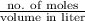
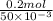
![Mg(OH)_{2} \rightleftharpoons Mg^{2+} + 2[OH^{-}]](/tpl/images/0210/7309/b229d.png)
![K_{sp} = [Mg^{2+}][OH^{-}]^{2}](/tpl/images/0210/7309/b67d6.png)
![4 \times [OH^{-}]^{2}](/tpl/images/0210/7309/cb444.png)
![[OH^{-}]](/tpl/images/0210/7309/e46dd.png) = 1.80276 M
= 1.80276 M![K_{w} = [H_{3}O^{+}][OH^{-}]](/tpl/images/0210/7309/912a0.png) ......... (1)
......... (1)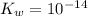
![[OH^{-}] = \frac{K_{w}}{[H_{3}O^{+}]}](/tpl/images/0210/7309/47cf2.png)
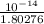
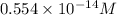
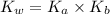 =
= 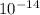
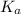 =
= 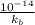
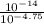
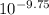
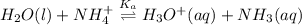
![K_{a} = \frac{[H_{3}O^{+}[NH_{3}]]}{[NH^{+}_{4}]}](/tpl/images/0210/7309/251f5.png) ....... (2)
....... (2)![[NH^{+}_{4}]](/tpl/images/0210/7309/286e5.png) as follows.
as follows.![\frac{[H_{3}O^{+}][NH_{3}]}{K_{a}}](/tpl/images/0210/7309/e4886.png)