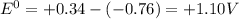Chemistry, 30.08.2019 00:30 mixcolin0002
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc sulfate. explain why there will be no reaction when zinc metal and aqueous copper sulfate solution are combined. identify the anode and the cathode, assuming a voltaic cell is constructed. note: be careful in the calculation of the standard cell potential ( eo cathode - eo anode). do not change the sign of the given reduction potential. the sign is already taken care of using the formula for calculating the standard cell potential.
standard reduction potential: cu2+(aq) + 2e- > cu(s) eo = -0.34 v
zn2+(aq) + 2e- > zn(s) eo = -0.76 v
standard cell potential = eo cathode - eo anode
although , combination of the reactants do not result in voltaic cells, it can be predicted if the reaction is spontaneous, based on the standard reduction potential.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc s...
Questions


Health, 03.03.2021 18:40

Chemistry, 03.03.2021 18:40

Computers and Technology, 03.03.2021 18:40

Mathematics, 03.03.2021 18:40



Mathematics, 03.03.2021 18:40

Biology, 03.03.2021 18:40



Mathematics, 03.03.2021 18:40

English, 03.03.2021 18:40

Physics, 03.03.2021 18:40

History, 03.03.2021 18:40

Geography, 03.03.2021 18:40

Computers and Technology, 03.03.2021 18:40

Mathematics, 03.03.2021 18:40


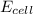 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous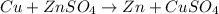
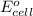 = standard electrode potential =
= standard electrode potential =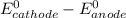
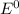 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Cu^{2+}/Cu]}= +0.34V](/tpl/images/0210/3859/55543.png)
![E^0_{[Zn^{2+}/Zn]}= -0.76V](/tpl/images/0210/3859/6c8c3.png)
![E^0=E^0_{[Zn^{2+}/Zn]}- E^0_{[Cu^{2+}/Cu]}](/tpl/images/0210/3859/f1898.png)
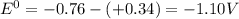
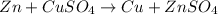
![E^0=E^0_{[Cu^{2+}/Cu]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0210/3859/cb03b.png)