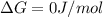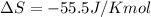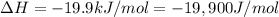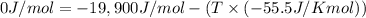Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
For a given reaction, ah = -19.9 kj/mol and as = -55.5 j/k/mol. the reaction will have ag = 0 at k....
Questions

Arts, 02.03.2021 05:20


Mathematics, 02.03.2021 05:20

Arts, 02.03.2021 05:20



Chemistry, 02.03.2021 05:20

Mathematics, 02.03.2021 05:20



History, 02.03.2021 05:20


Chemistry, 02.03.2021 05:20







Arts, 02.03.2021 05:20

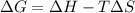
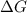 = Change in Gibbs free energy
= Change in Gibbs free energy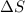 = Change in an entropy
= Change in an entropy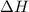 = Enthalpy of reaction
= Enthalpy of reaction