
Chemistry, 29.08.2019 16:30 toricepeda82
Consider the following equilibrium. 2 so2 (g) o2 (g) 2 so3 (g) the equilibrium cannot be established when is/are placed in a 1.0-l container. a) 0.75 mol so2 (g) b) 0.25 mol of so2 (g) and 0.25 mol of so3 (g) c) 1.0 mol so3 (g) d) 0.50 mol o2 (g) and 0.50 mol so3 (g) e) 0.25 mol so2 (g) and 0.25 mol o2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Consider the following equilibrium. 2 so2 (g) o2 (g) 2 so3 (g) the equilibrium cannot be established...
Questions


English, 29.09.2019 08:50




Chemistry, 29.09.2019 08:50




Computers and Technology, 29.09.2019 08:50





Mathematics, 29.09.2019 08:50

Biology, 29.09.2019 08:50


Chemistry, 29.09.2019 08:50


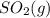
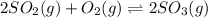
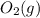 to form
to form 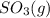 .
.

