
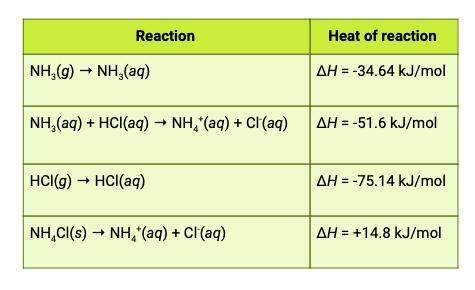
The heat of reaction for the process described in (a) can be determined by applying hess's law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data. which two of these heats of reaction would be the easiest and safest to measure in the laboratory, and which two are better obtained through reference sources? why? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases.
this is the equation for a: δh25° = ( δh25° nh3 + δh25° hcl) - ( δh25° nh4cl)
and attached is the table

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
The heat of reaction for the process described in (a) can be determined by applying hess's law. the...
Questions


Mathematics, 22.10.2020 20:01

History, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01



Mathematics, 22.10.2020 20:01

History, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01

History, 22.10.2020 20:01






Mathematics, 22.10.2020 20:01



