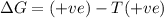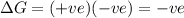For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one would predict thata. δh˚ is negative and δs˚ is negativeb. δh˚ is positive and b. δs˚ is negativec. δh˚ is positive and δs˚ is positived. δh˚ is negative and δs˚ is positive

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1
You know the right answer?
For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one wou...
Questions

Mathematics, 16.01.2021 19:50


Mathematics, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50

Chemistry, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50

History, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50

World Languages, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50


Mathematics, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50



Mathematics, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50

Social Studies, 16.01.2021 19:50

Mathematics, 16.01.2021 19:50

Biology, 16.01.2021 19:50

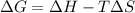
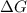 = Gibbs free energy
= Gibbs free energy 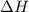 = enthalpy change
= enthalpy change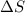 = entropy change
= entropy change 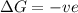
 >
> 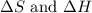 both have positive values.
both have positive values.