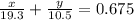Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, respectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold (by mass) in the jewelry.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

You know the right answer?
Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry cont...
Questions




Computers and Technology, 16.08.2021 20:40


Engineering, 16.08.2021 20:40





Social Studies, 16.08.2021 20:40



Social Studies, 16.08.2021 20:40




English, 16.08.2021 20:40