
Chemistry, 28.08.2019 04:30 kingcory717
505 grams of koh are required to completely react with 4.50 mole of sulfuric acid. how many moles of products are produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1


You know the right answer?
505 grams of koh are required to completely react with 4.50 mole of sulfuric acid. how many moles of...
Questions

Social Studies, 10.07.2019 04:10



History, 10.07.2019 04:10


Biology, 10.07.2019 04:10


History, 10.07.2019 04:10

History, 10.07.2019 04:10

Mathematics, 10.07.2019 04:10

History, 10.07.2019 04:10



Biology, 10.07.2019 04:10

Social Studies, 10.07.2019 04:10

History, 10.07.2019 04:10


Biology, 10.07.2019 04:10


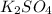 and
and 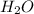 are, 4.50 and 9 moles.
are, 4.50 and 9 moles.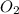 = 32 g/mole
= 32 g/mole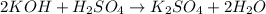
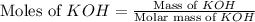
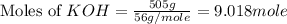
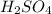 react with 2 mole of
react with 2 mole of 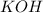
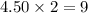 moles of
moles of 

