Chemistry, 28.08.2019 03:10 jonmorton159
A0.020 m solution of niacin has a ph of 3.26. (a) what percentage of the acid is ionized in this solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

You know the right answer?
A0.020 m solution of niacin has a ph of 3.26. (a) what percentage of the acid is ionized in this sol...
Questions

Chemistry, 26.11.2019 19:31

Mathematics, 26.11.2019 19:31


Mathematics, 26.11.2019 19:31



Mathematics, 26.11.2019 19:31



Biology, 26.11.2019 19:31

Mathematics, 26.11.2019 19:31

Mathematics, 26.11.2019 19:31


History, 26.11.2019 19:31


History, 26.11.2019 19:31


History, 26.11.2019 19:31


Biology, 26.11.2019 19:31

![pH=-log [H+]](/tpl/images/0204/3941/a7279.png)
![3.26 = -log [H+]](/tpl/images/0204/3941/b8b1f.png)
![[H+] = 5.495\times 10^{-4} M](/tpl/images/0204/3941/5b1d5.png)
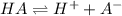
![K_a=\frac{[H+][A-]}{[HA]}](/tpl/images/0204/3941/c9423.png)
![K_a=\frac{[x][x]}{[0.020-x]}](/tpl/images/0204/3941/20ef9.png)
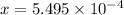
![K_a=\frac{[5.495\times 10^{-4}]^2}{[0.020-5.495\times 10^{-4}]}](/tpl/images/0204/3941/3796a.png)
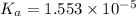
![\frac{[H^+_eqm]}{[Acid_{initial}]}\times 100](/tpl/images/0204/3941/b38c0.png)