
Chemistry, 27.08.2019 23:30 ryleepretty
Three students made multiple weighings of a copper cylinder, each using a different balance. the correct mass of the cylinder had been previously determined to be 47.32 g. describe the accuracy and precision of each student's
measurements.
mass of cylinder (g)
lissa lamont leigh anne
weighing 1 47.13 47.45 47.95
weighing 2 47.94 47.39 47.91
weighing 3 46.83 47.42 47.89
weighing 4 47.47 47.41 47.93

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
Three students made multiple weighings of a copper cylinder, each using a different balance. the cor...
Questions

Computers and Technology, 28.11.2020 05:50

Mathematics, 28.11.2020 05:50





History, 28.11.2020 05:50

Chemistry, 28.11.2020 05:50








English, 28.11.2020 05:50

History, 28.11.2020 05:50

Chemistry, 28.11.2020 05:50

Computers and Technology, 28.11.2020 05:50

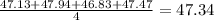 . The average is close to the true value of 47.32 and hence is accurate.The results are not close to each other hence the measurement is not precise. Thus Lissa's are measurements are accurate but not precise.
. The average is close to the true value of 47.32 and hence is accurate.The results are not close to each other hence the measurement is not precise. Thus Lissa's are measurements are accurate but not precise.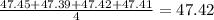 . The average is not close to the true value of 47.32 and hence is not accurate. The results are close to each other hence the measurement is precise.Thus Lammont's measurements are not accurate but precise.
. The average is not close to the true value of 47.32 and hence is not accurate. The results are close to each other hence the measurement is precise.Thus Lammont's measurements are not accurate but precise.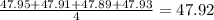 . The average is not close to the true value of 47.32 and hence is not accurate. The results are close to each other hence the measurement is precise.Thus Leigh Anne's measurements are not accurate but precise.
. The average is not close to the true value of 47.32 and hence is not accurate. The results are close to each other hence the measurement is precise.Thus Leigh Anne's measurements are not accurate but precise.

