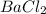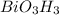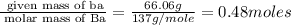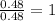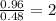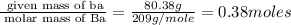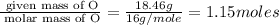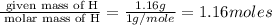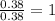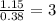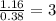Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Determine the empirical formula for compounds that have the following analyses: a. 66.0% barium and...
Questions

Mathematics, 07.04.2021 20:00

Mathematics, 07.04.2021 20:00


English, 07.04.2021 20:00

Chemistry, 07.04.2021 20:00


Mathematics, 07.04.2021 20:00






Mathematics, 07.04.2021 20:00

Mathematics, 07.04.2021 20:00

Computers and Technology, 07.04.2021 20:00


Mathematics, 07.04.2021 20:00