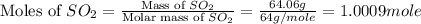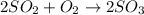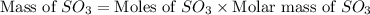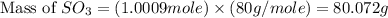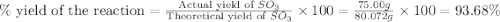In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulfur trioxide. using the equation, 2so2 (g) + o2 imported asset 2so3 (g), if 64.06g of sulfur dioxide is given an opportunity to react with an excess of oxygen to produce 75.00 g of sulfur trioxide, what is the percent yield of this reaction? 46.83% 60.25% 75.55% 93.68%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1

Chemistry, 23.06.2019 11:20
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1

Chemistry, 23.06.2019 15:10
Certain types of organisms such as fireflies and anglerfish can produce light through chemical reactions in a process called bioluminescence. what kind of chemical reactions occur during bioluminescence? o a) exothermic ob) endothermic oc) recomposition od) decomposition
Answers: 2
You know the right answer?
In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulf...
Questions







Mathematics, 20.04.2021 22:40

Computers and Technology, 20.04.2021 22:40

Mathematics, 20.04.2021 22:40

English, 20.04.2021 22:40



Advanced Placement (AP), 20.04.2021 22:40

English, 20.04.2021 22:40


Arts, 20.04.2021 22:40


Mathematics, 20.04.2021 22:40


English, 20.04.2021 22:40

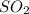 = 64.06 g
= 64.06 g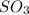 = 80 g/mole
= 80 g/mole