
Chemistry, 27.08.2019 19:10 robinsonchristopher0
Onsider the following unbalanced chemical equation for the reaction which is used to determine blood alcohol levels: h1+ + cr2o72- + c2h6o → cr3+ + co2 + h2o in the forward reaction, is chromium oxidized and/or reduced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Onsider the following unbalanced chemical equation for the reaction which is used to determine blood...
Questions

History, 03.12.2021 22:20

Mathematics, 03.12.2021 22:20



Mathematics, 03.12.2021 22:20

Mathematics, 03.12.2021 22:20

Mathematics, 03.12.2021 22:20

Social Studies, 03.12.2021 22:20







Biology, 03.12.2021 22:20


Biology, 03.12.2021 22:20



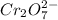 = +6
= +6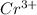 = +3
= +3

