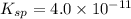Chemistry, 27.08.2019 18:00 erinharrington15
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the...
Questions

Mathematics, 19.04.2021 17:20



Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20




Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20


Chemistry, 19.04.2021 17:20


Mathematics, 19.04.2021 17:20

English, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20

English, 19.04.2021 17:20


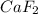 will form its respective ions in the solution as:
will form its respective ions in the solution as: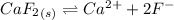
![[Ca^{2+}][F^-]^2](/tpl/images/0203/0217/bfb98.png)
![[Ca^{2+}]=0.049\ M](/tpl/images/0203/0217/5b4bb.png)
![[F^-]=0.147\ M](/tpl/images/0203/0217/c9382.png)