
Chemistry, 27.08.2019 17:20 catt707p38uu0
Argon (molecular weight 40 g/mole) is a monatomic compound. if liquid argon is confined to a container and held at a constant temperature of 80.5 k, what is the approximate vapor pressure of gaseous argon, assuming the liquid has no entropy and a binding energy of 0.1 ev? [note: at 1 atm, the boiling point is 87.3 k.]

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Argon (molecular weight 40 g/mole) is a monatomic compound. if liquid argon is confined to a contain...
Questions

Computers and Technology, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

Social Studies, 17.04.2021 14:00


Mathematics, 17.04.2021 14:00




History, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

English, 17.04.2021 14:00

English, 17.04.2021 14:00

English, 17.04.2021 14:00


Mathematics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00


Mathematics, 17.04.2021 14:00

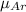 =
= 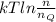
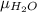 =
= 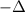
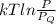 =
= 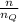 =
= 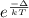
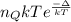
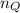 =
= 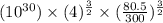
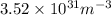
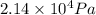 .
.

